Fourier transform infrared Hadamard tomography of sooting flames
Stephen C. Bates, Kim Knight
Thoughtventions Unlimited LLC, 40 Nutmeg Lane, Glastonbury, CT 06033
Robert Carangelo, and Michael Serio
Advanced Fuel Research, 87 Church Street, East Hartford, Connecticut 06108
ABSTRACT
An experimental technique is described that combines tomography, Hadamard signal encodement, and a patented Fourier transform infrared (FT-IR) emission/transmission (E/T) technique to perform simultaneous spatially resolved gas species and soot measurements during combustion. Tomographic analysis of line-of-sight FT-IR data allows spatially resolved measurements to be made. Hadamard encodement of the tomographic sections increases the overall signal throughput, improving the signal to noise (S/N) ratio for each measurement. The Hadamard technique leads to a major simplification in the tomographic apparatus in that the scanning apparatus that would normally be required is eliminated, and focusing of the infrared light is much easier. An experiment demonstrating Hadamard data processing as applied to FT-IR tomography is described. Deconvolution of the encoded data is shown to be accurate and gives the predicted improvement in S/N ratio. The FT-IR Hadamard tomography is performed to measure soot in a fuel-rich diffusion flame. Spatially resolved concentration measurements agree well with previous data, and clearly show striking three-dimensional features that could not normally be measured by simple line-of-sight techniques.
1. INTRODUCTION
There are a wide variety of techniques currently available as combustion diagnostics. Laser spectroscopic and scattering techniques as well as other optical diagnostics have been developed to measure gas species concentration and gas temperature, as well as soot particle size and concentration. Many of these techniques were reviewed by Penner et al. [1,2] and Hardesty, and some have been applied to study soot formation in gaseous flames. [4-13] Several new two-dimensional (2-D) flow visualization techniques have been developed using lasers. [14-20] These and other new 2-D techniques were recently reviewed by Hanson. [21] The completeness of a simultaneous data set of the temperature, composition, and physical properties of the different gas species and soot is critical for exact process control and research into understanding the complex reaction system of a combustor. Fourier transform infrared (FT-IR) spectroscopy provides a means for obtaining this data set using a single instrument. Competing diagnostic techniques utilize lasers in different ways, but these diagnostics are typically difficult to use and expensive.
One of the most important processes within a hydrocarbon combustion environment is the formation and evolution of soot. Soot measurement provides a direct monitor of combustion inefficiency, radiation heat losses, and particulate pollution. Also, since soot is such a strong radiator in flames, and soot and gas temperatures are often significantly different, both of these temperatures must be measured separately. Most studies of soot formation have been performed in flames. Extensive measurements in diffusion flames show that soot is formed primarily in the fuel-rich regions where pyrolysis is occurring. [4-10,12,22,23] A substantial amount of work (especially on surface growth) has also been performed in atmospheric pressure two-dimensional premixed flames using compounds beginning with ethylene and progressing through a series of more automatic compounds.[ 24-26] Advances in the understanding and measurement of the formation and destruction of soot must be made before improved combustion devices can be designed and operated. Detailed simultaneous measurements of the concentration and temperature of soot and gas species are needed, especially of soot precursors and soot combustion products.
II. FT-IR EMISSION/TRANSMISSION (E/T) TOMOGRAPHY
A FT-IR emission/transmission (E/T) spectroscopic technique for diagnosing gas concentration and temperature was recently developed and patented (US No. 4,653,766) by Advanced Fuel Research (AFR). [27-34] The FT-IR soot diagnostic is built around a Bomem Michelson spectrometer. This instrumentation has a resolution of 4 cm-1 using a comer cube mirror system and a Ge-KBr beam splitter that allows data acquisition from 6500 cm-1 (1.5 μm) to 400 cm-1 (25 μm). The spectrometer has been modified for sequential emission/transmission FT-IR to be able to perform simultaneous measurement of the temperature, composition, and physical properties of the different gas species and soot. A similar FT-IR sp ectrometer system together with IR collection optics and detectors was developed and used to perform process measurements during hazardous waste incineration at the SERI High Flux Solar Furnace in Golden, CO, in a recovery boiler for paper waste recycling at the Weyerhauser facility in Longview, WA, and in a flue gas stream from a coal-fired power plant in Beverly, OH.
A. Emission/transmission spectroscopy
The background for the FT-IR E/T tomography is described by work performed on line-of-sight E/T spectroscopy. [27-34] For multiphase reacting systems, measurements are made of the transmittance and the radiance, and from these a normalized radiance is calculated. For non-scattering optically thin samples such as gas and soot, the quantity I -transmittance is proportional to the total extinction from each absorbing component. The radiance is proportional to the extinction times the Planck black body function as a function of wavelength. The normalized radiance, which is the radiance divided by (1 - transmittance), is then proportional to the Planck black body function at the temperature of the component. This proportionality applies across the spectra of black and gray bodies such as soot, as well as at the specific absorption wavelengths of a particular gas. The complete analysis for non-thin samples and for particles follows Siegel and Howell [35] and has been presented previously. [31]
B. Transmission tomography
An integrating line path measurement can be used to tomographically reconstruct a fully three-dimensional image of a partially absorbing object. The three-dimensional images are formed by combining a closely spaced parallel set of spatially resolved two-dimensional image slices through the object. These two-dimensional images are in turn mathematically reconstructed using tomography from integral line-of-sight measurements that span a plane through the object, where the beam must pass through each area element from two different directions. A beam measurement that is scanned in a plane perpendicular to the beam (up and down, left and right) to cover an entire measurement volume can be used to reconstruct a 3-D image of an axially symmetric object. For the creation of 3-D images, both the required absorption measurements and the reconstruction itself are greatly simplified for objects with this axial symmetry. The most common reconstruction techniques in these cases use a radial inversion based on Abel's equations. [36--39] More recent reconstruction techniques can produce two-dimensional slices of an image for objects of arbitrary shape. [40-43]
For transmission tomography, the basic measurements are line-of-sight absorbances. The spatial dependencies of both gas concentrations and temperatures have been obtained previously from tomography applied to monochromatic measurements of a steady-state flame. [44] These results apply to gas samples without a condensed phase. The simultaneous presence of a condensed phase (e.g., soot) and other gas band features reduces the accuracy of the monochromatic measurements. The effects of soot can only be separated when the image reconstruction techniques described above are based on local measurements and include IR absorbance spectra over an extended wave-number range. Accurate local concentrations can then be determined for soot and all of the gases present in the flame. At this time, only the case of a low absorbance sample (transmittance > 80%) has been studied in this manner. [22]
C. Emission tomography
A straightforward application of reconstruction techniques to scans of radiance is not possible where there is self-absorption in the sample. There are a number of cases for which the self-absorbance has been corrected to obtain local species concentrations and temperatures from line-of-sight emission and transmission measurements. In the case of small absorbance (transmission > 80%), an emission measurement can be corrected by an absorption measurement made along the same path. A radial inversion can be applied directly to the emission thus corrected to obtain local radiances. [45]
For samples with larger absorbance (80% >percent transmission > 20%) an iterative procedure in conjunction with radial inversion has been used to give local radiances. [44] In addition, in those cases for which there is not a linear relationship between absorbance and concentration (Beer's Law does not apply), a band model in conjunction with radial inversion has led to the determination of local gas species concentrations and temperatures. [46,47]
In the work described below, the flame had high transmission so that the radiance could be corrected for self-absorption using the direct method. [45] The Fourier reconstruction technique was applied to these corrected radiance spectra to give local radiances. These reconstruction procedures were applied to each 16 cm- 1 wave-number interval from 500-6500 cm-1 to build up local emission and transmission IR spectra. The analysis of these local spectra was performed in the same manner as for thin homogenous samples.
D. Standard tomography results
Spectral tomography for an ethylene diffusion flame has been described previously. [27] In these measurements, [21] parallel scans were made at each of ten different heights in the flame. The beam diameter in these cases was 1 min. For each height the transmission scans were converted to absorbance ( -log10 T, where T is the transmittance), since it is the projections of this quantity across the flame which are the appropriate inputs to image reconstruction algorithms. A Fourier reconstruction of the two-dimensional images from the projections was performed using the program published by Shepp and Logan. [44]
Local spectra were obtained for transmittance, radiance, and normalized radiance. From these data, concentrations and temperatures were obtained for the soot, as well as for CO2, H2O, alkanes, alkenes, and alkynes. The results of the local soot concentrations were obtained from the percent transmittance at 6500 cm-1 using the optical constants for soot measured by Dalzell and Sarofim [48] These values are compared with laser extinction and scattering data obtained by Santoro et al. [49] for a flame formed by the same burner under nominally identical conditions with very good agreement between the results obtained from the two different techniques. Temperature comparisons were made with the thermocouple measurement of gas temperatures (lumped) by Santoro et al.[49] and the non-intrusive CARS temperature measurement for CO2 by Boedeker and Dobbs.[13]
The temperatures from the FT-IR tomography were about 100°C higher than those from thermocouple measurement, with an additional 100°C increment in the soot zone. On the other hand, there is excellent agreement between FT-IR and CARS. It is most probable that radiation heat loss from soot-covered thermocouples is the cause of low temperatures in the thermocouple measurements.
Reconstructed images of organics, Soot, CO2, and H2O concentrations were obtained as well as the temperature profiles for the same species. One conclusion from this data is that average soot and gas temperatures in the same local pixel (1 mm3) can have significantly differing values. The differences cross several pixel boundaries in a region of slowly varying temperatures, and so cannot be an artifact of the measurement. While contrary to previous comments on the matter,[50] these observations are the first experimental evidence on the relative local soot and gas temperatures. The FT-IR tomography has demonstrated the unique capability of measuring local species concentrations and temperatures in mixed phase systems.
III. HADAMARD PROCESSING
Hadamard processing was originally developed as a means of increasing the optical throughput of a dispersive spectrometer. In front of the detector is placed a mask that has a specific combination of open and closed slots. Light impinges on the detector through all of the open slots. A data set is formed by using a series of masks that have slot combinations that change in a pattern determined by the Hadamard encodement. The traditional spectral data for each slot is derived from this data set by postprocessing using the Hadamard deconvolution formulas. The advantage of this procedure is that because each measurement taken through the mask passes through multiple slots it has an improved signal to noise (S/N) ratio, which leads to an improved S/N ratio of the deconvolved data relative to single slot measurements. The Hadamard encoding mask replaces the spectrometer exit slit with an aperture that is either reflective (or opaque) or transmissive to chosen spectral elements; the spectral elements allowed to impinge upon the detector are determined by the Hadamard encodement. The Hadamard encodement is also cyclic, so that the series of masks can be replaced by a linearly translating mask. Once this mask has gone through its complete cycle and all patterns of the Hadamard encodement matrix have been in front of the aperture, the detector responses as a function of the Hadamard encodement number are converted by the Hadamard transform into a conventional spectrum.
Hadamard transform spectroscopy (HTS) has been described in depth by several authors. [51-57] For a stationary Hadamard encoding mask in single detector HTS, the number of slots is N for N spectral elements. The Hadamard encodement matrix is cyclic, such that a moving Hadamard encoding mask is made with 2N-1 slots for N spectral elements and translated across the N-wide opening to give the proper N different slot combinations. The cyclic arrangement of open and closed elements in an array of size N is determined by a cyclic matrix of order N. A total of N individual Hadamard encodements are required for N data acquisitions before the Hadamard transform can be performed to obtain spectral data. Each of the N Hadamard encodements has (N+1)/2 slots that transmit their spectral element and (N-1)/2 slots that reject their spectral element. The number of spectral elements incident on the single detector is thus as close to half of those present as is possible for an odd number of spectral elements.
In this case the multiplexing advantage in FITS is achieved by allowing approximately half of the spectral elements to impinge upon a single detector for each measurement. A general treatment of the multiplexing advantage has been presented elsewhere [58,59] and shows that the improvement in signal-to-noise ratio (S/N) is given by the expression (NS+1)/(2√NS), where NS = the number of scans for Fourier transform spectroscopy (FTS) or the number of spectral elements for HTS. The factor for improvement in S/N approaches √NS/2 as NS increases for HTS compared to the corresponding factor of √N-S for FTS. Thus, the factors for both FTS and HTS increase in proportion in. √NS. In HTS the S/N for NS = 7 spectral resolution elements increases by a factor of 1.51 and for NS = 127 the S/N increases by a factor of 5.68 compared to dispersive spectrometry.
Hadamard transform spectrometers have been successfully developed for use in a variety of applications. These include photothermal deflection imaging, [60] infrared spectroscopy, [61] two-dimensional Hadamard transform infrared imaging, [51] Fourier transform infrared imaging [52] and other spectroscopic instrumentation. [53-55] The utility of Hadamard processing is usually based on the multiplex advantage found in the data acquisition and the simplicity of the application of the technique. Optical spectrometric methods and thermal detection have probably been the main focus as a result of the substantial improvement in the S/N afforded by the multiplex capability. Consequently, a significant effort has been devoted toward the development of these optical Hadamard transform instruments.
IV. ADVANTAGES OF FT-IR HADAMARD TOMOGRAPHY
Hadamard processing improves the S/N ratio of FT-IR tomography measurements through the multiplexing advantage discussed above. The greater S/N of added signals is combined with the noise averaging of multiple measurements to give deconvolved measurements that have greater S/N than each measurement taken singly. A Hadamard measurement also greatly reduces focusing losses because the beam covers the entire mask rather than having to be focused down to the area of a single slit. Focusing on a single slit would otherwise be necessary to achieve the same spatial resolution. The large illuminated area is important for FT-IR applications because a typical FT-IR spectrometer beam is large and poorly collimated. Although half of the infrared probe beam is thrown away on the closed Hadamard mask elements, this loss can be recovered using fiberoptics to perform the encoding as discussed below.
The other major advantage of Hadamard tomography is the simplification of the tomographic measurements. This occurs because normally to build up the tomographic sections the infrared beam would have to be moved across the flame with a scanning apparatus. In Hadamard tomography, the complex scanning equipment is replaced with Hadamard masks whose elements provide the appropriate slices through the flame. Furthermore, the cyclic character of the Hadamard encoding permits a single translating, 2N-1-element-wide mask moving across an N-wide opening to be used instead of separate masks for each encodement. The FT-IR beam is sized optically to fill the N-wide opening, which in turn is sized to include all or part of the flame as desired. A set of Hadamard measurements are made and the tomographic slices are deconvolved using the Hadamard inverse matrix. Given the computational power already associated with the FT-IR measurements, Hadamard deconvolution processing is a minor additional requirement.
V. HADAMARD MEASUREMENT DEMONSTRATION
Feasibility experiments were done to demonstrate the process of Hadamard manipulation in the context of tomography using transparent plastic to simulate an infrared spectrum. Shown in Fig. 1 is the overall Hadamard mask and the encoding masks, designed with seven elements. Seven was chosen as the minimum number of elements that could resolve the flame profile with minimum processing complexity (the next largest permissible number of Hadamard elements is 14). The moving mask is 13 (2N-1) elements wide and sized vertically to give the appropriate tomographic resolution in that direction.
A uniformly thick, visually transparent plastic was placed in front of the mask and sized so that it covered the entire area of element 4 in the seven element wide mask. The plastic remained fixed as the mask and the encodement changed, generating an artificial spectra in element 4. The seven FT-IR Hadamard spectra are shown in Fig. 2, and they appear to differ in only minor ways. In spite of this, the seven measurements are deconvolved using the inverse Hadamard matrix to give the single slot spectra of Fig. 3, which clearly show the distinctive spectrum of the transparency isolated at position 4. Figure 4 shows a comparison of the deconvolved position 4 spectrum with a direct spectrum of the transparency; the match is excellent. The spectrum of the plastic sheet was taken over the open seven-wide aperture, so that it has much higher S/N, accounting for the difference in the intensity of the spectra.
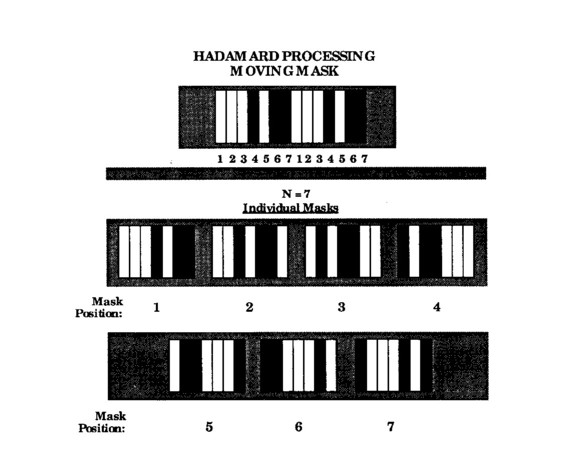
Fig. 1 Hadamard masking; single moving mask and individual encodement masks.
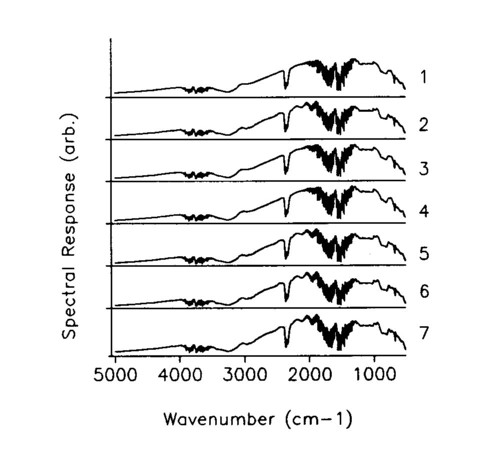
Fig. 2. Spectra measurements for each Hadamard mask encodement.
To verify the improved S/N of the Hadamard process, regions of the spectra where absorption was not significant were isolated, and the spectral noise was computed as a square root of the value squared at each wave number. The results are shown in Figs. 5a and 5b. The improvement in signal-to-noise is expected to be 1.5 based on the formula (NS+1)/(2√NS), and this is confirmed visually, although a numerical calculation is difficult as a result of the spectral variations. The S/N improvement increases approximately in proportion to √NS/2 for large NS.
The S/N improvements of the Hadamard process can be increased by using fiber optics. Although the Hadamard process increases S/N by increasing the light throughput for each measurement, the blocking elements of the mask necessarily limit the usage of the beam to slightly over half the beam area. This is not an inherent limitation because fiber optics can be used to transform the entire beam area to the desired Hadamard encodement by distributing the output fibers in the pattern required for each encoding position. In this case, the S/N increases by another factor of 2 to become (NS+1)/√NS, a very significant improvement, especially for a smaller number of elements. Such a fiber optic encodement could be done by translating slot-width bundles back and forth within the overall mask to form the appropriate Hadamard combinations.
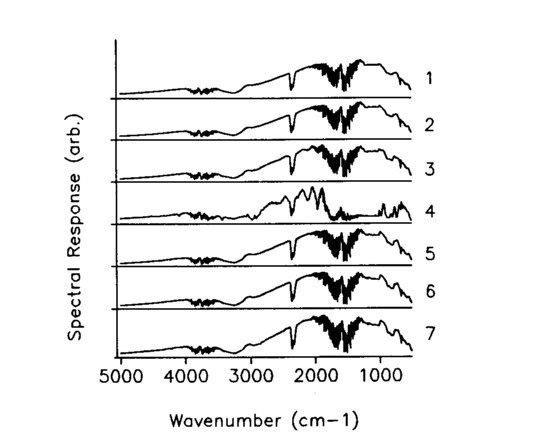
FIG. 3. Deconvolved spectra for each of the seven single slots of the mask encodement.
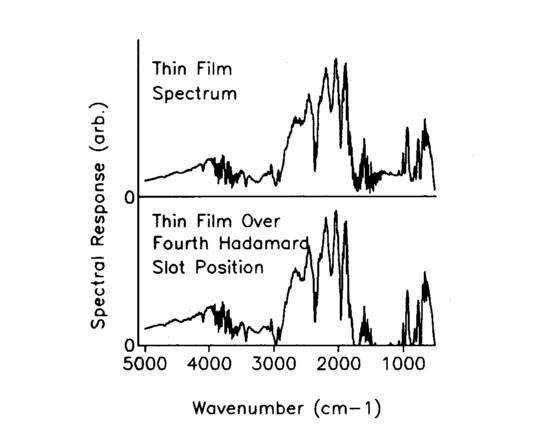
FIG. 4. Comparison of the thin film spectral response through a sevenwide opening with the convolved spectrum of the fourth single slot position covered by the film.
VI. FT-IR HADAMARD TOMOGRAPHY MEASUREMENTS IN AXISYMMETRIC FLAMES
The FT-IR ERNST Hadamard tomography was validated in a co-annular laminar ethylene diffusion flame of the type studied at the National Institute of Science and Technology (NIST) [4,5,8,10] and elsewhere. [6,7,13] Extensive data on soot formation and species concentration has been obtained in these flames by other methods. Tomography on this flame has been performed previously in a project at AFR 39 and has been described above as Standard Tomography Results. For the work described here a flame of similar height and flows (here; 3.65 cm3/s fuel) was used. The diffusion flame was stabilized with a lexan tube with slots for FIP-IR beam access. A restriction was also placed at the top of the enclosure beneath the exhaust duct. Within this enclosure the flame was stable to within a few tenths of a millimeter and axisymmetric.
The primary emphasis was on concentration measurements, which were derived from transmission spectra through the flame. A FT-IR absorbance spectrum of the fuel-rich part of the flame is shown in Fig. 6 to show all of the readily identifiable species in the system. Absorption features characteristic of ethylene (C2H4), carbon dioxide
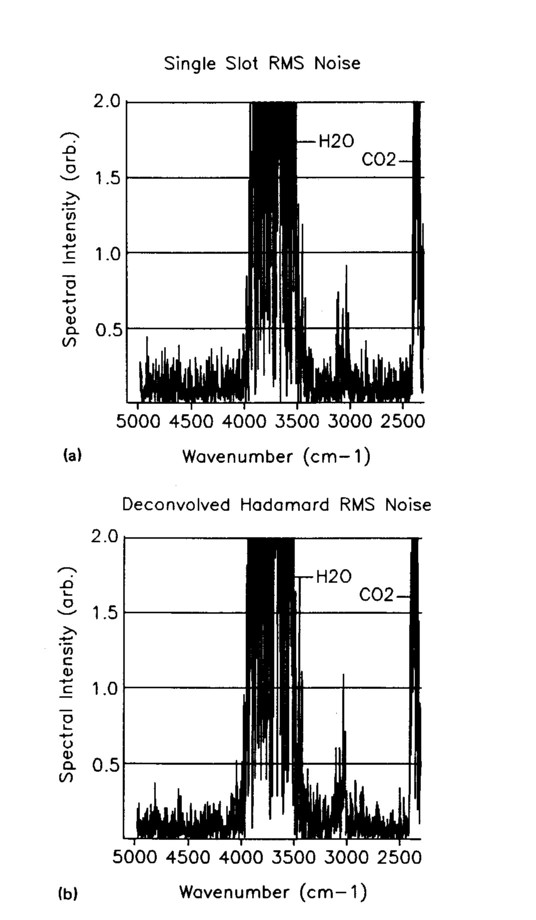
FIG. 5. The Fr-IR spectral root-mean-squared (rms) noise (a) measured through a single slot and (b) measured as a single-slot Hadamard deconvolution
(CO2), carbon monoxide (CO), and acetylene (C2H2) are shown in the figure. An absorbance spectrum through the sooting part of the flame is shown in Fig. 7. The signature of the soot presence is seen from the sloping baseline. Concentration measurements were determined for CO2 Using the peak at 668 cm-1; for acetylene using its primary peak at 730 cm-1; for CO using an integral under the curve of the upper branch of the absorption centered at 2145 cm-1; and for soot by measuring the absorbance value at 4000 cm-1 as described in previous work. Gas concentration calibrations were performed at AFR using commercial calibration gases and a similar FT-IR 4 cm-1 resolution spectrometer. Care must be taken in comparing the room temperature calibration spectra with those taken through the flame. The wings of the absorption bands spread out and decrease as more states are occupied as a result of the higher energy at higher temperature.
The FT-IR beam was focused (condensed) through the mask and flame with parabolic mirrors, leading to much improved S/N compared with a full-sized, but masked, spectrometer beam. A moving Hadamard mask based on seven elements was made out of strips of aluminum foil attached to a stainless steel sheet.
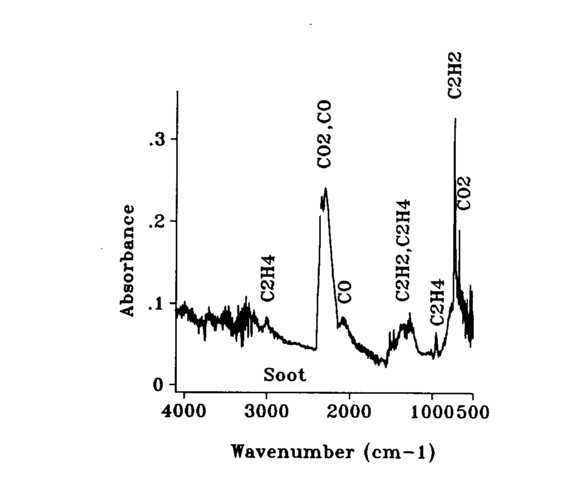
Fig. 6. The FT-IR transmission spectrum through an ethylene-oxygen diffusion flame.
Each element was 1.0 mm wide and 7.0 mm high; the mask coding was checked with a reticle to be accurate to 0.1 mm or better in element positioning. The mask was placed 1.5 cm from the flame center to minimize IR beam divergence effects in the distance between the mask and the flame. Making the mask with 1-mm wide elements was difficult, but necessary to be the appropriate size to span the ethylene diffusion flame. A typical industrial application will use larger masks. Moving the mask with precision was also difficult to do by hand but will be simple in an actual instrument, which will use a stepper motor.
The tomography on the flame was performed with the seventh (end) mask element centered at the center of the flame, diving the flame into seven radial segments. Five adjacent vertical positions were scanned starting from a position where the mask was centered 11 mm above the fuel inlet, so that only the base of the flame is measured. Background measurements were alternated with flame measurements for each mask position to minimize thermal distortion effects. After a set of Hadamard measurements were completed at one vertical position, transmission spectra were computed using the background spectrum for each appropriate mask position. The seven Hadamard mask measurements were then deconvolved using the Hadamard inverse matrix to give single slot data. The single slot data was then deconvolved tomographically to give relative concentration as a function of radius.
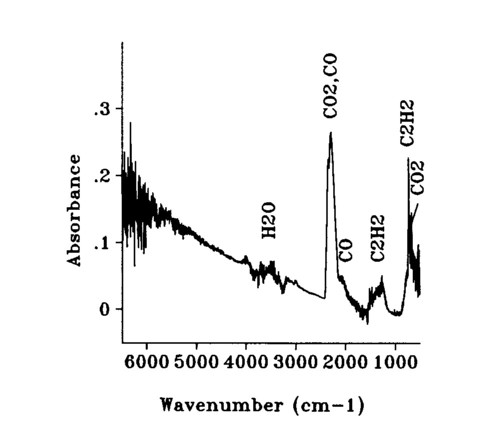
FIG. 7. The FT-IR transmission spectrum through the sooting region of the ethylene-oxygen flame.
Species measured were the ethylene fuel, acetylene (a soot precursor), soot, and CO2 ; the data are presented in Figs. 8a-8d. The line-of-sight measurements are reconstructed radially, and information about the central value arises only from one cord measurement passing through the center. For this reason the S/N is very low at the center and the data are presented showing the central value extrapolated based on the data trends. This makes the data easier to understand visually. These data are not intended to be precise, only to demonstrate the power of Hadamard FT-IR tomography. Each data point represents an average concentration value over the 1 x 7-mm slot.
Figures 8a -8d show a great amount of detail in the flame structure. The data is comparable to the lower half of the flame images presented from earlier work. Figure 8a shows the ethylene fuel fed into the base of the flame and being consumed. Figure 8b shows the soot precursor acetylene being formed from the hot ethylene and being consumed by the soot formation process. Figure 8c shows the soot being formed in an outer shell and at the center as the ethylene pyrolyses. Figure 8d shows the CO2 being formed as the soot bums. The sections do not include all of the flame, as is shown by the soot peaks at the greatest radius in Fig. 8c.
The concentration data shows a major feature that does not appear in the previous data. The ethylene, acetylene, and soot data indicate that there is a toroidal region in the flame with accentuated soot production. Since this was not expected, the literature was consulted to determine if this was a real effect. In fact, Santoro et a1 [4] have found this region in their research, describing the soot formation process that causes it in detail. Our data confirms his results. The soot data presented in Fig. 8(c) is somewhat misleading as a result of an artifact of the graphics that draws descending contours in a different manner than ascending contours. This accounts for the asymmetry in the figure even though the data is symmetric (by definition), but obscures the toroidal region in the soot data. The spatially resolved concentration results agree well with previous data, and clearly show striking 3-D features that could not normally be measured by simple line-of-sight techniques.

Tomographically deconvolved temperature measurements were also made at one height in the flame by taking emission data and calibrating with a blackbody source. Figure 9 shows the results, giving the soot and CO2 temperatures across the flame at an average height of 25 mm in the flame. Once again, the central measurements are unreliable, so they have been omitted. It can be seen that the CO2 temperature is considerably higher that the soot temperature, as expected, since the soot radiates so much energy.
Finally, experiments were done to determine if soot concentration changes dependent on fuel doping could be followed. Figure 10 shows the variation in soot concentration with the fraction of acetylene in the ethylene fuel feed. The increased slope of the spectrum is directly proportional to concentration and shows a global increase in soot in the flame averaged across the entire flame as the fraction of ethylene is increased. Both oxygen and hydrogen were also added, but the change in soot production was much less.
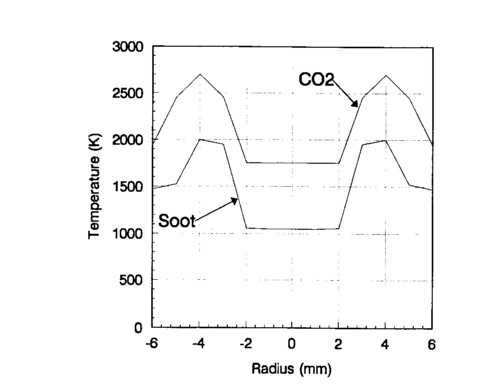
FIG. 9. Ethylene-air diffusion flame soot and CO2 temperature profiles derived from Hadamard tomography measurements.
VII. APPLICATIONS
Since FT-IR Hadamard tomography can provide spatially resolved volume measurements of both concentration and temperatures of gas species and soot simultaneously, it is expected to have broad applicability to the study of complex combustion problems. The method will be especially valuable to sooting systems, since it can also detect the concentration and temperature of species important to soot formation, such as acetylene and polyaromatic hydrocarbons.
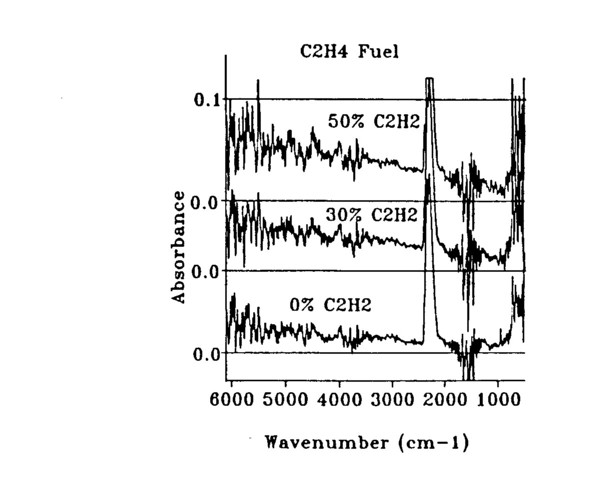
FIG. 10. Ethylene-air diffusion flame soot variation with acetylene replacing ethylene fuel.
Potential applications in combustion process control include furnaces, boilers, and incinerators, which would all benefit from the application of this monitoring system to achieve increased operating efficiency. Applications in pollution monitoring are widespread, ranging from measuring the output of soot itself as particles that are dangerous to breathe, to the optimization of combustion processes for minimizing pollutant production. In research, the system has many applications as a pure measurement device, and other applications where by being able to measure soot and soot precursor gases simultaneously it can greatly enhance soot science. In all these applications the FT-IR system has the advantage of making the same measurements with a single instrument that previously required a number of separate devices.
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation under Small Business Innovative Research (SBIR) Grant No. ISI-9060897, and the authors wish to express their thanks to the agency and the program that made this work possible.
REFERENCES
1. S. S. Penner, C. P. Wang, and M. Y. Bahadori, Prog. Energy Comb. Sci. 10, 209 (1984).
2. S S. Penner, C. P. Wang, and M. Y. Bahadori, 20th Symp. (Int) Comb. (The Combustion Institute, Pittsburgh, PA, 1984), p. 1149.
3. D. R. Hardesty, Report No. SAND84-8724, Sandia National Laboratories ( 1984).
4. R. J. Santoro and H. G. Semerjian, and R. A. Dobbins, Combust. Flame 51, 203 (1983).
5. R. J. Santoro and H. G. Semerjian, The 20th Symp. (Int) on Comb. (The Combustion Institute, Pittsburgh, PA, 1984), p. 997.
6. J. H. Kent and H. Gg. Wagner, The 20th Symp. (Int) Comb. (The Combustion Institute, Pittsburgh, PA, 1984), p. 1007.
7. G H. Markstein and J. debris, The 20th Sym. (Int) Comb. (The Combustion Institute Pittsburgh, PA, 1984), p. 1637.
8. R. J. Santoro, T. T. Yeh, and H. G. Semerjian, in Heat Transfer in Fire and Combustion Systems, edited by C. K. Law, Y. Jaluria, W. W. Yuen, and K. Miyasaka (American Society of Mechanical Engineering, New York, 1985), p. 57.
9. K. C. Smyth, J. H. Miller, R. C. Dorfman, W. G. Mallard, and R. J. Santoro, Combust. Flame 62, 157 (1985).
10. R. J. Santoro, T. T. Yeh, J. J. Horvath, and H. G. Semerjian, The 1985 Fall Technical Meeting, Eastern Section of the Combustion Institute, Nov. 4-6, 1985, Philadelphia, PA.
11. A. C. Eckbreth and R. J. Hall, Combust. Flame 36, 87 (1979).
12. W. L. Flower, C. T. Bowman, 20th Symp. (Int.) on Comb. (The Combustion Institute, Pittsburgh, PA, 1984), p. 1035.
13. L. R. Boedeker and G. M. Dobbs, Combust. Sci. and Tech. 36:3-6, 301 (1986).
14. L. D. Chen, W. M. Roquemore, Combust. Flame 66, 81 (1986).
15. W M. Roquemore, R. S. Tankin, H. H. Chui, and S. A. Lottes, Exp. Fluids 4, 205 (1986).
16. M. B. Long, B. T. Chu, and R. K. Cheng, AIAAA J. 19,1151 (1981).
17. M. C. Escoda and M. B. Long, AIAA J. 21, 81 (1983).
18. M B. Long, P. S. Levin, and D. C. Fourguette, Opt. Lett. 10, 267 (1985).
19. T Utami and T. Ueno, Exp. Fluids 2, 25 (1984).
20. M G. Allen, R. D. Howe, and R. K. Hanson, Opt. Lett. 11 (3), 126 (1986).
21. R. K. Hanson, 21st Symposium (Int) on Combustion, Invited Paper.
22. P. E. Best, P. L. Chien, R. M. Carangelo, and P. R. Solomon, Combust. Flame 85, 309 (1991).
23. K. C. Smyth and J. H. Miller, Science 236, 1540 (1987).
24. S J. Harris and A. M. Weiner, 20th Symp. (Int) on Comb. (The Combustion Institute, Pittsburgh, PA, 1984), p. 969.
21. S J. Harris, A. M. Weiner, R. J. Blint, and J. E. M. Goldsmith, 21st Symp. (Intl on Comb. (The Combustion Institute Pittsburgh, PA, 1986), p. 1033.
26. L Baumgartner, D. Hesse, H. Jander, and H. G. G. Wagner, 20th Symp. (Int) on Comb. (The Combustion Institute, Pittsburgh, PA, 1984), p. 959.
27. P. R. Solomon, P. E. Best, R. M. Carangelo, J. R. Markham, P. L. Chien, R. J. Santoro, and H. G. Semedian, 21st Symp. (Int.) Comb. (The Combustion Institute Pittsburgh, PA, 1986), p. 1763.
28. P. R. Solomon, M. A. Serio, R. M. Carangelo, and J. R. Markham, Fuel 65, 182 (1986).
29. P. R. Solomon, R. M. Carangelo, D. G. Hamblen, and P. E. Best, Appl. Spectrosc. 40, 6, 746 (1986).
30. P R. Solomon, R. M. Carangelo, P. E. Best, J. R. Markham, and D. G. Hamblen, Fuel 66, 897 (1987).
31. P. E. Best, R. M. Carangelo, J. R. Markham, and P. R. Solomon, Combust. and Flame 66, 47 (1986).
32. P. R. Solomon, R. M. Carangelo, P. E. Best, J. R. Markham, and D. G. Hamblen, 21st Symp. (Int) on Comb. (The Combustion Institute, Pittsburgh, PA, 1996), p. 437.
33. P. R. Solomon, R. M. Carangelo, P. E. Best, J. R. Markham, D. G. Hamblen, and P. L. Chien, in Fundamentals of Physical-Chemistry of Pulverized Coal Combustion, edited by J. Lahaye and G. Prado (Kluwer, New York, 1987), p. 347.
34. P. R. Solomon, P. L. Chien, R. M. Carangelo, P. E. Best, J. R. Markham, 22nd Symp. (Int) on Comb. (The Combustion Institute, Pittsburgh, PA, 1988), p. 211.
35. R. Siegel and J. R. Howell, Thermal Radiation Heat Transfer (McGraw-Hill, New York, 1972).
36. R. H. Tourin, Spectroscopic Gas Temperature Measurement (Elsevier, New York, 1966).
37. W. J. Pearce, Conference on Extremely High Temperatures (Wiley, New York, 1958), pp. 123-135.
38. B. J. Hughey and D. A. Santavicca, Combust. Sci. Tech. 29, 167 (1982).
39. C. B. Ludwig, W. Malkums, J. R. Reardon, and J. A. L. Thomson, in Handbook of Infrared Radiation from Combustion Gases, edited by R. Goulard and J. A. L. Thompson NASA SP-3080 (NASA, Scientific and Technical Information Office, Washington, DC, 1973).
40. S. Ray and H. G. Semerjian, "Laser Tomography for Simultaneous Concentration and Temperature Measurements in Reacting Flows," AIAA 18th Thermophysics Conference, Montreal, Canada (1983).
41. R. J. Santoro, H. G. Semedian, P. J. Emmerman, and R. Gouland, Int. J. Heat Mass Transfer 24 (7), 1139 (1981).
42. H. G. Semedian, R. J. Santoro, R. Goulard, and P. J. Emmerman, in Fluid Mechanics of Combustion Systems, edited by T. Morel, R. P. Lohmann, and J. M. Rackley (The American Society of Mechanical Engineers, New York, 1981), p. 119.
43. L. A. Shepp and B. F. Logan, IEEE Trans. Nucl. Sci. NS-21, 21 (1974).
44. P. Elder, T. Jerrick, and J. W. Birkeland, Appl. Opt. 4 (5), 589 (1965).
45. M P. Freeman and S. J. Katz, J. Opt. Soc. Am. 50 (8), 826 (1960).
46. C. C. Limbaugh in Infrared Methods for Gaseous Measurement: Theory and Practice, edited by J. Wormhoudt (Marcel Dekker, New York, 1985), Chap. 5, p. 197.
47. L. E. Brewer and C. C. Limbaugh, Appl. Opt. 11, 1200 (1972).
48. W. H. Dalzell, and A. J. Sarofim, Heat Transfer 92, 100 (1969).
49. R J. Santoro, T. T. Yeh, and H. G. Semerjian, Conference on Lasers and Electro-Optics Baltimore, MD, 21 May 1985 (Optical Society of America, 1985), p. 300.
50. C. Millikan, in Temperature, It's Measurement and Control in Science and Industry, Vol. 3, Part 2, edited by A. 1. Dahl (Reinhold, New York, 1962 p. 497.
51. H. Coufal, U. Moller, and S. Schneider, Appl. Opt. 21, 116 (1982).
52. M. E. Kraenz and D. Kunath, J. Molec. Struct. 79, 47 (1982).
53. N. Sugimoto, Appl. Opt. 25, 863 (1986).
54. F. W. Plankey, T. H. Glenn, L. P. Hart, and J. D. Winefordner, Anal. Chem. 46, 1000 (1974).
55. M. Harwit, Appl. Opt. 10, 1415 (1971).
56. R. M. Hammaker, J. A. Graham, D. C. Tilotta, and W. G. Fateley, Vibrational Spectra and Structure, Vol, 15, edited by J. R. Durig (Elsevier, Amsterdam, 1986), pp. 401-485.
57. R. D. Swift, R. B. Wattson, J. A. Decker, Jr., R. Paganetti, and M. Harwit, Appl. Opt. 15, 1595 (1976).
58. A P. Bohlke, J. D. Tate, J. S. White, J. V. Paukstelis, R. M. Hammaker, and W. G. Fateley, J. Molec. Struct. (Theochem.) 200, 471 (1989).
59. S. A. Dyer and J. B. Park, Appl. Spectrosc. 43, 278 (1989).
60. F. K. Fotiou and M. D. Morris, Appl. Spectrosc. 40, 704 (1986).
61. J. A. Decker, Jr., Appl. Opt. 21, 116 (1982).